Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Collaborative Laboratories for Advanced Decommissioning Science; Tokyo Institute of Technology*
JAEA-Review 2022-028, 54 Pages, 2022/11
The Collaborative Laboratories for Advanced Decommissioning Science (CLADS), Japan Atomic Energy Agency (JAEA), had been conducting the Nuclear Energy Science & Technology and Human Resource Development Project (hereafter referred to "the Project") in FY2021. The Project aims to contribute to solving problems in the nuclear energy field represented by the decommissioning of the Fukushima Daiichi Nuclear Power Station, Tokyo Electric Power Company Holdings, Inc. (TEPCO). For this purpose, intelligence was collected from all over the world, and basic research and human resource development were promoted by closely integrating/collaborating knowledge and experiences in various fields beyond the barrier of conventional organizations and research fields. The sponsor of the Project was moved from the Ministry of Education, Culture, Sports, Science and Technology to JAEA since the newly adopted proposals in FY2018. On this occasion, JAEA constructed a new research system where JAEA-academia collaboration is reinforced and medium-to-long term research/development and human resource development contributing to the decommissioning are stably and consecutively implemented. Among the adopted proposals in FY2019, this report summarizes the research results of the "Development of Tailor-made Adsorbents for Uranium Recovery from Seawater on the Basis of Uranyl Coordination Chemistry" conducted from FY2019 to FY2021. Since the final year of this proposal was FY2021, the results for three fiscal years were summarized. The present study aims to develop a new ligand class for efficient and selective capture of uranium from seawater. On the basis of deep understanding on uranyl coordination chemistry, we design molecular structures of pentadentate ligands as functional moieties for uranium adsorption from seawater and study fundamental coordination chemistry of uranyl ion with those ligands in order to resolve current problems in uranium recovery technology …
 and Eu
and Eu ions onto sedimentary rock in the presence of gamma-irradiated humic acid
ions onto sedimentary rock in the presence of gamma-irradiated humic acidZhao, Q.*; Saito, Takeshi*; Miyakawa, Kazuya; Sasamoto, Hiroshi; Kobayashi, Taishi*; Sasaki, Takayuki*
Journal of Hazardous Materials, 428, p.128211_1 - 128211_10, 2022/04
Times Cited Count:5 Percentile:62.11(Engineering, Environmental)The influence of humic acid and its radiological degradation on the sorption of Cs and Eu
and Eu by sedimentary rock was investigated to understand the sorption process of metal ions and humic substances. Aldrich humic acid (HA) solution was irradiated with different doses of gamma irradiation using a Co-60 gamma-ray source prior to the contact between the metal ions and the solid sorbent. The HA molecule decomposed to smaller molecules with a lower complexation affinity. Batch sorption experiments were performed to evaluate the effect of gamma-irradiated HA on the sorption of Cs
by sedimentary rock was investigated to understand the sorption process of metal ions and humic substances. Aldrich humic acid (HA) solution was irradiated with different doses of gamma irradiation using a Co-60 gamma-ray source prior to the contact between the metal ions and the solid sorbent. The HA molecule decomposed to smaller molecules with a lower complexation affinity. Batch sorption experiments were performed to evaluate the effect of gamma-irradiated HA on the sorption of Cs and Eu
and Eu ions. The addition of non-irradiated HA weakened the sorption of Eu because of the lower sorption of the neutral or negatively charged Eu-HA complexes compared with free Eu ions. The sorption of monovalent Cs ions was barely affected by the presence of HA and its gamma irradiation. The concentration ratio of HA complexed species and non-complexed species in the solid and liquid phases was evaluated by sequential filtration and chemical equilibrium calculations. The ratios supported the minimal contribution of HA to Cs sorption. However, the concentration ratio for Eu
ions. The addition of non-irradiated HA weakened the sorption of Eu because of the lower sorption of the neutral or negatively charged Eu-HA complexes compared with free Eu ions. The sorption of monovalent Cs ions was barely affected by the presence of HA and its gamma irradiation. The concentration ratio of HA complexed species and non-complexed species in the solid and liquid phases was evaluated by sequential filtration and chemical equilibrium calculations. The ratios supported the minimal contribution of HA to Cs sorption. However, the concentration ratio for Eu in the liquid phase was high, indicating that the complexing ability of HA to Eu
in the liquid phase was high, indicating that the complexing ability of HA to Eu was higher than that of HA to Cs
was higher than that of HA to Cs ions. Therefore, the sorption of free Eu
ions. Therefore, the sorption of free Eu would predominate with the gamma irradiation dose applied to the HA solution under a radiation field near the HLW package.
would predominate with the gamma irradiation dose applied to the HA solution under a radiation field near the HLW package.
Collaborative Laboratories for Advanced Decommissioning Science; Tokyo Institute of Technology*
JAEA-Review 2021-041, 42 Pages, 2022/01
The Collaborative Laboratories for Advanced Decommissioning Science (CLADS), Japan Atomic Energy Agency (JAEA), had been conducting the Nuclear Energy Science & Technology and Human Resource Development Project (hereafter referred to "the Project") in FY2020. The Project aims to contribute to solving problems in the nuclear energy field represented by the decommissioning of the Fukushima Daiichi Nuclear Power Station, Tokyo Electric Power Company Holdings, Inc. (TEPCO). For this purpose, intelligence was collected from all over the world, and basic research and human resource development were promoted by closely integrating/collaborating knowledge and experiences in various fields beyond the barrier of conventional organizations and research fields. The sponsor of the Project was moved from the Ministry of Education, Culture, Sports, Science and Technology to JAEA since the newly adopted proposals in FY2018. On this occasion, JAEA constructed a new research system where JAEA-academia collaboration is reinforced and medium-to-long term research/development and human resource development contributing to the decommissioning are stably and consecutively implemented. Among the adopted proposals in FY2019, this report summarizes the research results of the "Development of tailor-made adsorbents for uranium recovery from seawater on the basis of uranyl coordination chemistry" conducted in FY2020. On the basis of deep understanding on uranyl coordination chemistry, we design molecular structures of pentadentate ligands as functional moieties for uranium adsorption from seawater and study coordination chemistry of uranyl ion with those ligands in order to resolve current problems in uranium recovery technology from seawater and to develop novel selective and efficient adsorbents for this purpose.
Sugiura, Yuki; Ishidera, Takamitsu; Tachi, Yukio
Applied Clay Science, 200, p.105910_1 - 105910_10, 2021/01
Times Cited Count:6 Percentile:51.59(Chemistry, Physical)Collaborative Laboratories for Advanced Decommissioning Science; Tokyo Institute of Technology*
JAEA-Review 2020-026, 41 Pages, 2020/12
The Collaborative Laboratories for Advanced Decommissioning Science (CLADS), Japan Atomic Energy Agency (JAEA), had been conducting the Nuclear Energy Science & Technology and Human Resource Development Project (hereafter referred to "the Project") in FY2019. The Project aims to contribute to solving problems in the nuclear energy field represented by the decommissioning of the Fukushima Daiichi Nuclear Power Station, Tokyo Electric Power Company Holdings, Inc. (TEPCO). For this purpose, intelligence was collected from all over the world, and basic research and human resource development were promoted by closely integrating/collaborating knowledge and experiences in various fields beyond the barrier of conventional organizations and research fields. The sponsor of the Project was moved from the Ministry of Education, Culture, Sports, Science and Technology to JAEA since the newly adopted proposals in FY2018. On this occasion, JAEA constructed a new research system where JAEA-academia collaboration is reinforced and medium-to-long term research/development and human resource development contributing to the decommissioning are stably and consecutively implemented. Among the adopted proposals in FY2019, this report summarizes the research results of the "Development of Tailor-Made Adsorbents for Uranium Recovery from Seawater on the Basis of Uranyl Coordination Chemistry". On the basis of deep understanding on uranyl coordination chemistry, we design molecular structures of pentadentate ligands as functional moieties for uranium adsorption from seawater and study coordination chemistry of uranyl ion with those ligands in order to resolve current problems in uranium recovery technology from seawater and to develop novel selective and efficient adsorbents for this purpose.
Yamaguchi, Tetsuji; Ohira, Saki; Hemmi, Ko; Barr, L.; Shimada, Asako; Maeda, Toshikatsu; Iida, Yoshihisa
Radiochimica Acta, 108(11), p.873 - 877, 2020/11
Times Cited Count:7 Percentile:66.68(Chemistry, Inorganic & Nuclear)Rai, D.*; Kitamura, Akira
Journal of Chemical Thermodynamics, 114, p.135 - 143, 2017/11
Times Cited Count:9 Percentile:19.61(Thermodynamics)Isosaccharinic acid is a cellulose degradation product that can form in low-level nuclear waste repositories and is known to form strong complexes with many elements, including actinides, disposed of in these repositories. We (1) reviewed the available data for deprotonation and lactonisation constants of isosaccharinic acid, and the isosaccharinate binding constants for Ca, Fe(III), Th, U(IV), U(VI), Np(IV), Pu(IV), and Am(III), (2) summarized complexation constant values for predicting actinide behavior in geologic repositories in the presence of isosaccharinate, and (3) outlined additional studies to acquire reliable thermodynamic data where the available data are inadequate.
 (am) in the aqueous K
(am) in the aqueous K - HCO
- HCO
 - CO
- CO
 - OH
- OH - H
- H O system
O systemRai, D.*; Kitamura, Akira; Rosso, K.*
Radiochimica Acta, 105(8), p.637 - 647, 2017/08
Times Cited Count:2 Percentile:19.65(Chemistry, Inorganic & Nuclear)Solubility of HfO (am) was determined as a function of KHCO
(am) was determined as a function of KHCO concentrations ranging from 0.001 mol.kg
concentrations ranging from 0.001 mol.kg to 0.1 mol.kg
to 0.1 mol.kg . The solubility of HfO
. The solubility of HfO (am) increased dramatically with the increase in KHCO
(am) increased dramatically with the increase in KHCO concentrations, indicating that Hf(IV) makes strong complexes with carbonate. Thermodynamic equilibrium constants for the formation of Hf-carbonate complexes were determined using both the Pitzer and SIT models. The dramatic increase in Hf concentrations with the increase in KHCO
concentrations, indicating that Hf(IV) makes strong complexes with carbonate. Thermodynamic equilibrium constants for the formation of Hf-carbonate complexes were determined using both the Pitzer and SIT models. The dramatic increase in Hf concentrations with the increase in KHCO concentrations can best be described by the formation of Hf(OH-)
concentrations can best be described by the formation of Hf(OH-) (CO
(CO )
)
 and Hf(CO
and Hf(CO )
)
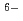 . The log
. The log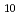 K
K values for the reactions [Hf
values for the reactions [Hf + 2 CO
+ 2 CO
 +2 OH
+2 OH
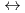 Hf(OH)
Hf(OH) (CO
(CO )
)
 ] and [Hf
] and [Hf + 5 CO
+ 5 CO

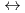 Hf(CO
Hf(CO )
)
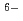 ], based on the SIT model, were determined to be 44.53
], based on the SIT model, were determined to be 44.53  0.46 and 41.53
0.46 and 41.53  0.46, respectively.
0.46, respectively.
 on Na-montmorillonite studied by time-resolved laser fluorescence spectroscopy and surface complexation modeling
on Na-montmorillonite studied by time-resolved laser fluorescence spectroscopy and surface complexation modelingSasaki, Takayuki*; Ueda, Kenyo*; Saito, Takumi; Aoyagi, Noboru; Kobayashi, Taishi*; Takagi, Ikuji*; Kimura, Takaumi; Tachi, Yukio
Journal of Nuclear Science and Technology, 53(4), p.592 - 601, 2016/04
Times Cited Count:12 Percentile:74.83(Nuclear Science & Technology)The influences of pH and the concentrations of Eu and NaNO
and NaNO on the sorption of Eu
on the sorption of Eu to Na-montmorillonite were investigated through batch sorption measurements and time-resolved laser fluorescence spectroscopy (TRLFS). The pH had a little effect on the distribution coefficients (Kd) in 0.01 M NaNO
to Na-montmorillonite were investigated through batch sorption measurements and time-resolved laser fluorescence spectroscopy (TRLFS). The pH had a little effect on the distribution coefficients (Kd) in 0.01 M NaNO , whereas the Kd strongly depended on pH at 1 M NaNO
, whereas the Kd strongly depended on pH at 1 M NaNO . A cation exchange model combined with a one-site non-electrostatic surface complexation model was successfully applied to the measured Kd. The TRLFS spectra of Eu
. A cation exchange model combined with a one-site non-electrostatic surface complexation model was successfully applied to the measured Kd. The TRLFS spectra of Eu sorbed were processed by parallel factor analysis (PARAFAC), which corresponded to one outer-sphere (factor A) and two inner-sphere (factor B and C) complexes. It turned out that factors A and B correspond to Eu
sorbed were processed by parallel factor analysis (PARAFAC), which corresponded to one outer-sphere (factor A) and two inner-sphere (factor B and C) complexes. It turned out that factors A and B correspond to Eu sorbed by ion exchange sites and inner-sphere complexation with hydroxyl groups of the edge faces, respectively. Factor C became dominant at relatively high pH and ionic strength and likely correspond to the precipitation of Eu(OH)
sorbed by ion exchange sites and inner-sphere complexation with hydroxyl groups of the edge faces, respectively. Factor C became dominant at relatively high pH and ionic strength and likely correspond to the precipitation of Eu(OH) on the surface.
on the surface.
Watanabe, Satoshi; Hashimoto, Kazuyuki; Ishioka, Noriko
JAEA-Review 2014-050, JAEA Takasaki Annual Report 2013, P. 102, 2015/03
Matsunaga, Takeshi; Nagao, Seiya*; Ueno, Takashi; Takeda, Seiji; Amano, Hikaru; Tkachenko, Y.*
Applied Geochemistry, 19(10), p.1581 - 1599, 2004/10
Times Cited Count:35 Percentile:55.27(Geochemistry & Geophysics)The association of dissolved  Sr,
Sr, 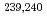 Pu and
Pu and 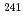 Am with natural colloids was investigated in surface waters in the Chernobyl nuclear accident area by means of ultrafiltration. Results suggest that Pu and Am isotopes were preferentially associated with dissolved humic substances (HS) of high molecular size. A model calculation of the complexation of Pu and Am with HS also supported the above. This study has expanded our understanding of the general role of natural organic colloids in dictating the chemical form of actinides in the surface aquatic environment.
Am with natural colloids was investigated in surface waters in the Chernobyl nuclear accident area by means of ultrafiltration. Results suggest that Pu and Am isotopes were preferentially associated with dissolved humic substances (HS) of high molecular size. A model calculation of the complexation of Pu and Am with HS also supported the above. This study has expanded our understanding of the general role of natural organic colloids in dictating the chemical form of actinides in the surface aquatic environment.
Nagao, Seiya*; Fujitake, Nobuhide*; Kodama, Hideki*; Matsunaga, Takeshi; Yamazawa, Hiromi
Journal of Radioanalytical and Nuclear Chemistry, 255(3), p.459 - 464, 2003/03
Times Cited Count:8 Percentile:49.89(Chemistry, Analytical)no abstracts in English
Tanaka, Tadao; Nagao, Seiya; Sakamoto, Yoshiaki; Ogawa, Hiromichi
Journal of Nuclear Science and Technology, 39(Suppl.3), p.524 - 527, 2002/11
Influence of humic acid on the sorption of Pu onto a coastal sand, which does not sorb humic acid, and an ando soil, which sorbs humic acid very well, was examined with respect to molecular sizes of humic acid. Sorption affinity of Pu for the coastal sand decreased with increasing humic acid concentration. As to the ando soil, the sorption affinity of Pu in the presence of humic acid was larger than that in the absence of humic acid, in low humic acid concentration range. These results suggest that apparent sorption affinity of Pu on the soils is dependent on the complexation ability with humic acid and the sorption affinity of the resulting humic complexes. Concentration profiles of Pu in each size fraction of solution before and after the sorption experiment were obtained by ultrafiltration technique. It was found that the complexation and sorption properties of humic acid are dependent on its molecular size and the important molecular size relating to the complexation and sorption properties tends to shift into smaller size ranges with increasing humic acid concentration.
Matsunaga, Takeshi
JAERI-Review 2001-018, 121 Pages, 2001/06
The present report reviews a series of studies conducted in JAERI which have dealt with the behavior of atmospherically-derived radionuclides in a fluvial environment. The studies cited here firstly include investigations of the evaluation of the transport rate of the atmospherically-derived 137Cs, 210Pb and 7Be from the ground via a river to the downstream areas where the affected water is consumed. The studies validated i) the importance of suspended particulate materials in the fluvial discharge of those radionuclides, and ii) a methodology to estimate the discharge of those radionuclides. Secondly, studies in rivers and lakes in the vicinity of the Chernobyl Nuclear Power Plant revealed the role of natural dissolved organics in affecting the dissolution and transport of 239,240Pu, 241Am through complexation to form soluble species with the aid of a chemical equilibrium model The same sort of a model was also applied successfully for the behavior of iron and manganese (hydr)oxides in river recharged aquifers which could bear riverborne radionuclides.
 Sr,
Sr, 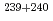 Pu and
Pu and 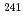 Am in the Sahan river waters from the Chernobyl area
Am in the Sahan river waters from the Chernobyl areaNagao, Seiya; Matsunaga, Takeshi; Fujitake, Nobuhide*; Amano, Hikaru
Proceedings of the International Workshop on Distribution and Speciation of Radionuclides in the Environment, p.162 - 168, 2000/00
no abstracts in English
Nagao, Seiya; Tanaka, Tadao; Sakamoto, Yoshiaki;
Radiochimica Acta, 74, p.245 - 249, 1996/00
no abstracts in English
Nagao, Seiya
Hoshasei Haikibutsu Kenkyu, 1(2), p.231 - 242, 1995/05
no abstracts in English
Nagao, Seiya*;
Science of the Total Environment, 117-118, p.439 - 447, 1992/00
no abstracts in English
 and lanthanide series elements
and lanthanide series elementsKamezawa, Akinori*; Suzuki, Shinichi; Kobayashi, Toru; Sueki, Keisuke*
no journal, ,
Kobayashi, Taishi*; Teshima, Takeshi*; Wang, P.*; Sasaki, Takayuki*; Kitamura, Akira
no journal, ,
We focused on the complexation of U(IV) and U(VI) with isosaccharinic acid (ISA) and measured U(IV) and U(VI) solubility in the presence of ISA. The solubility dependences on hydrogen ion concentration (pHc) and ISA concentration revealed the dominant U(IV) and U(VI) ISA complexes and determined their formation constants. Furthermore, the solubility of Zr(IV), as an analogous of An(IV), in the presence of organic acids with different number of hydroxyl groups were investigated to elucidate the strong complexation ability of ISA.